A combined approach to targeting the tumor and its microenvironment
Context
Glioblastomas are the most aggressive brain tumors and represent a major challenge in oncology. Originating from glial cells, they are characterized by rapid proliferation, diffuse infiltration into healthy brain tissue, and resistance to conventional treatments. Their impact on patients is devastating, leading to progressive neurological decline, cognitive impairments, and a significantly reduced life expectancy, with a median survival of just 15 months post-diagnosis despite current therapies.
The standard treatment relies on a multimodal approach combining surgery, radiotherapy, and chemotherapy with temozolomide. However, its effectiveness remains limited due to tumor infiltration, the blood-brain barrier, and therapy resistance. Tumor-Treating Fields (TTF) therapy has shown a modest survival benefit, but its high cost complicates widespread adoption, particularly in low-resource healthcare systems. Other therapeutic strategies are under investigation, including immunotherapy, gene therapy, and targeted nanoparticles, but their efficacy has yet to be confirmed in clinical trials.
Finding a treatment that extends both survival and quality of life for glioblastoma patients remains an urgent priority.
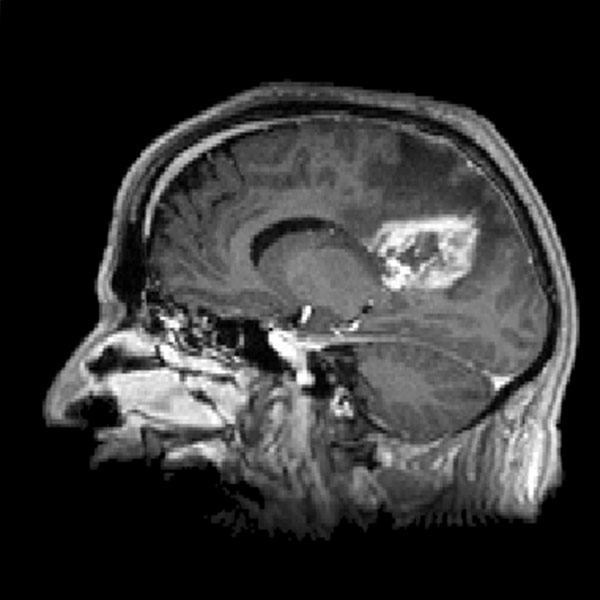
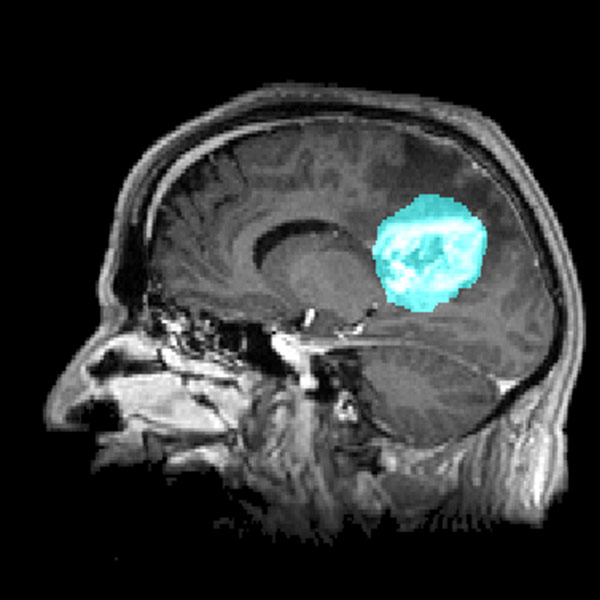
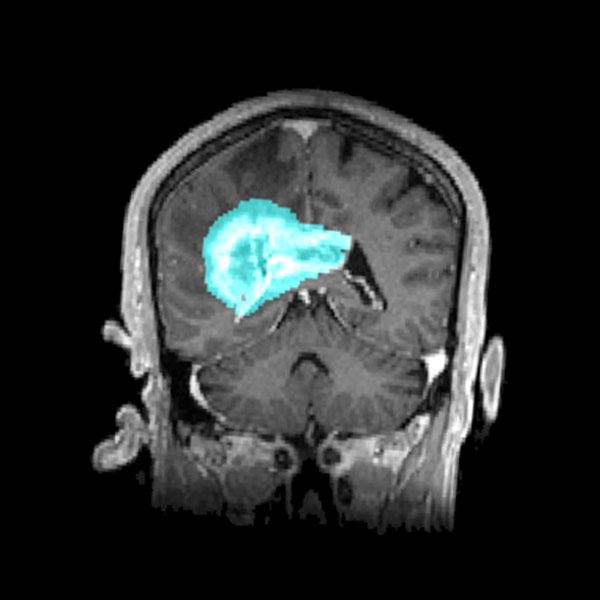
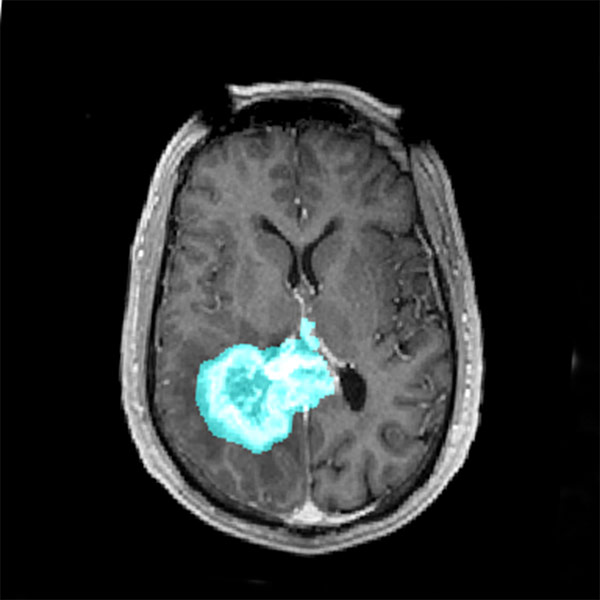
The tumor microenvironment plays a crucial role in glioblastoma progression but is often overlooked in therapy development. Recent studies have highlighted the close interaction between tumor cells and the neuronal environment, revealing that glioblastomas establish functional synaptic connections with surrounding neurons. This aberrant communication induces neuronal hyperactivity, which, in turn, fuels tumor proliferation and spread.
One well-known factor in this process is glutamate, which contributes to tumor microenvironment hyperexcitability. Glioblastoma cells excessively release this neurotransmitter, activating NMDA and AMPA receptors, thereby promoting proliferation, migration, and angiogenesis. This excitotoxicity also alters the activity of glial cells, particularly astrocytes, which release inflammatory molecules that reinforce a pro-tumor environment.
More recent studies (Barron, Tara, et al. 2025) have highlighted modifications in GABAergic signaling within the tumor microenvironment. Normally inhibitory in mature neurons, GABA (gamma-aminobutyric acid) becomes excitatory in tumor cells due to abnormal intracellular chloride accumulation. This shift results from the overexpression of NKCC1, a chloride importer, creating a vicious cycle where neuronal activity supports glioblastoma expansion rather than restraining it.
These findings underscore the importance of integrating the tumor microenvironment into therapeutic strategies. Targeting the communication between cancer cells and neurons—particularly by modulating GABAergic signaling—could open new avenues for slowing glioblastoma progression and enhancing current treatments.
Project
In response to this challenge, B&A Oncomedical, in collaboration with Neurochlore, has launched an innovative project based on a combination therapy approach. By acting not only on the tumor itself but also on its surrounding environment—a key driver of its progression—this strategy aims to offer a more effective and better-tolerated treatment for glioblastoma patients.
Bumetanide (BUM)
Bumetanide (BUM), a diuretic treatment, is gaining increasing interest in oncology.
Its mechanism of action is based on the inhibition of the NKCC1 co-transporter, a protein involved in ion flux regulation and highly overexpressed in glioblastoma tumor cells. By blocking NKCC1, Bumetanide reduces intracellular chloride accumulation, restoring the inhibitory effect of GABA and thereby mitigating neuronal hyperexcitability—a key factor in tumor progression.
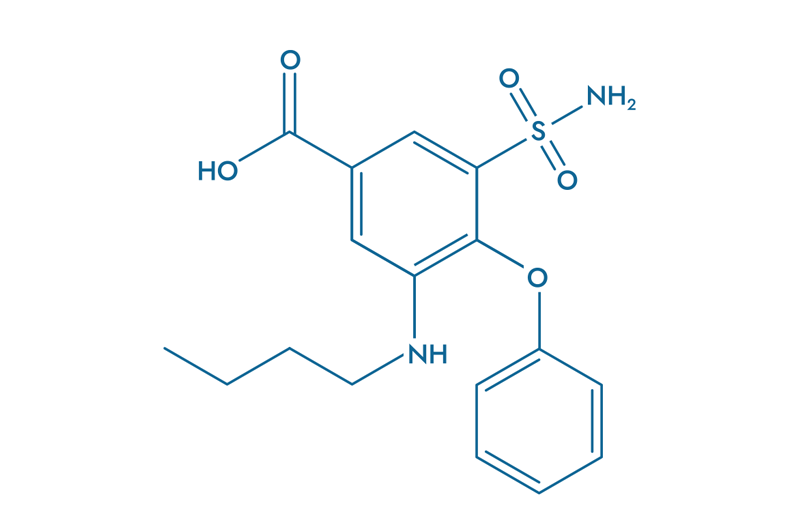
- Blocks neuronal hyperactivity
- Reduces tumor-related neuronal excitability
- Decreases interaction between neurons and cancer cells
- Prevents tumor migration
- Prevents metastasis
Mebendazole (MBZ)
Mebendazole (MBZ) is a well-known antiparasitic agent recognized for its ability to target microtubules.
By inhibiting microtubule polymerization, MBZ disrupts cell division and promotes apoptosis in cancer cells. This drug has already demonstrated anticancer efficacy through numerous experimental studies, as well as in a Phase I/II trial conducted on glioblastoma patients by a team from Johns Hopkins University (Gallia, Gary L., et al., 2021).
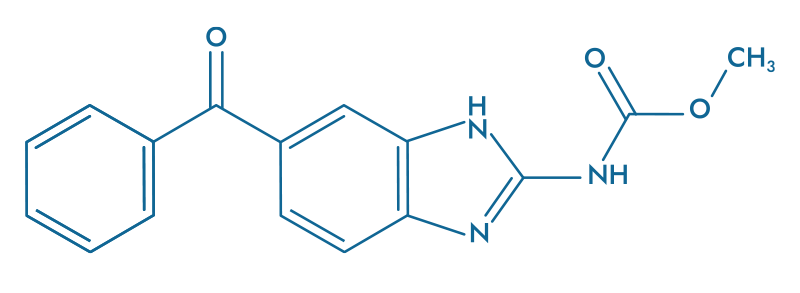
- Inhibits microtubule polymerization
- Induces apoptosis in tumor cells
- Prevents cancer cell division
- Demonstrated efficacy in Phase I/II glioblastoma trial
- Well-tolerated safety profile
Leveraging its diverse expertise in ex vivo and in vitro applications, B&A Oncomedical, in close collaboration with Prof. F. Berger and his team in Grenoble, has implemented cutting-edge methodologies to assess the therapeutic potential of this combination. These approaches include the use of 3D tumoroid cultures and on-chip models, as well as electrophysiological recordings on murine cortical slices alongside studies on freshly resected tumors—featuring, for the first time, direct measurements of individual GABA channels and intracellular chloride (Cl⁻) levels. By faithfully replicating the architecture and cellular interactions observed in vivo, these advanced models allow us to explore not only the direct effects of treatments on tumor cells but also the complex interactions within the tumor microenvironment, a critical factor in tumor progression and resistance.
Results and perspectives
Based on the experiments conducted by our teams, we have observed the potential of combining Mebendazole and Bumetanide in the treatment of glioblastoma.
By exploiting complementary cellular mechanisms, the COMBO reduces a tumor size and increases cell death within tumor cells.
Furthermore, considering the synaptic hyperexcitability, which is promoted by glioblastoma and responsible for the onset of epileptiform seizures, this combination has demonstrated anticonvulsant effects by blocking the hyperexcitability of cancer cells, even at low concentrations.
Finally, in a compassionate pilot trial, the administration of the combination in a patient showed a remarkable increase in life expectancy.
In conclusion, the combination of Mebendazole and Bumetanide presents a significant therapeutic interest for the treatment of glioblastoma. The COMBO not only reduces tumor migration and increases apoptosis of cancer cells but also acts synergistically at low doses on the hyperexcitability of the tumor microenvironment. This opens the door to new therapeutic strategies for this aggressive brain tumor.
These promising results, covered by a PCT application, pave the way for an innovative therapeutic approach targeting both tumor cells and their neuronal environment, with potential applications for other brain tumors. Our goal now is to secure funding and mobilize investors to launch a Phase 2/3 clinical trial evaluating the effectiveness of the BUM x MBZ combination for the treatment of glioblastomas and other brain tumors.






